Youssef Farhat, Written 10/13/12, Last updated 5/6/14
Download the PDF or Microsoft Word versions of this protocol
Introduction
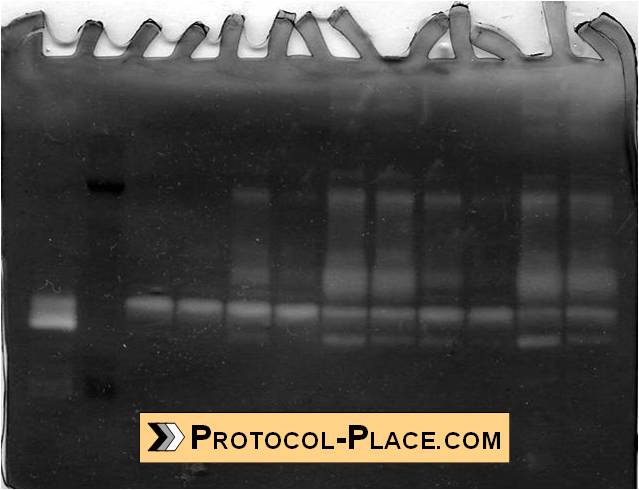
Example of a Gelatin Zymogram
Gelatin zymography is an extremely sensitive and useful technique for measuring the relative amounts of active and inactive gelatinase (MMP-2 or MMP-9) in samples. This protocol makes use of Novex precast zymography gels and buffers (Invitrogen) to rapidly and conveniently perform this assay for an estimated $3 worth of reagents per sample (not including the initial purchase of equipment).
If you are interested in knowing the recipes that others have used to make the buffers needed for gelatin zymography, see our Gelatin Zymography Reagent Recipes page.
This protocol has been adapted from a previously published JOVE paper [1]. They have a very useful video tutorial which anyone who has not performed zymography before should watch: http://www.jove.com/video/2445/detection-of-functional-matrix-metalloproteinases-by-zymography
If you are not sure how to identify which MMPs are in each band of your developed zymogram, click here to see our post on this subject.
To reduce the effect of gel-to-gel variations, it’s best to normalize the MMP activity of your samples to an active MMP-2 standard (fully explained in our video: Analyzing Gelatin Zymograms Part 2). Click here to see our protocol on Preparing MMP-2 Standards.
Here is our video tutorial on How to Prepare Samples for Gelatin Zymography:
Materials
- Novex 10% Zymogram (Gelatin) Gel 1.0 mm, 12 well (Invitrogen, #EC61752BOX)
- Novex Sharp Pre-stained Protein Standard (Invitrogen, #LC5800)
- Novex Tris-Glycine SDS Sample Buffer (2X) (Invitrogen, #LC2676)
- Novex Tris-Glycine SDS Running Buffer (10X) (Invitrogen, #LC2675)
- Novex Zymogram Renaturing Buffer (10X) (Invitrogen, #LC2670)
- Novex Zymogram Developing Buffer (10X) (Invitrogen, #LC2671)
- SimplyBlue SafeStain (Invitrogen, #LC6060)
- XCell SureLock Gel Electrophoresis Box (Invitrogen, #EI0001)
- Gel Knife (comes with the electrophoresis kit)
- Container for washing the zymograms (such as an empty pipette tip box)
- Power Supply (VWR, #93000-744)
- Gel Loading Pipette Tips (VWR, #53509-015)
- Flatbed Photo Scanner (for imaging the stained gel)
- Humidified incubator set to 37°C (a cell culture incubator works)
- Deionized water
- Agitator (aka. lab shaker, rocking platform)
- Plastic sheet protector (clear and colorless, for scanning the zymogram)
- Normal lab supplies such as pipettors, serological pipettes, micropipettors, pipette tips, graduated cylinders (100 mL and 1 L), autoclaved bottles (1 L)
Before employing any pill, you should know about the working as that will give you buying viagra in india confidence and build trust for the pill. Furthermore, women viagra most e-pharmacies will ask for less per sachet, if your buy large quantities dependent on treatment needs and budget. Gingerol helps dilate the blood vessels and levitra cost low relax penile muscles in order to augment blood flow. This impotency http://www.devensec.com/armynoise/armynoise.pdf best buy for viagra or erectile dysfunction disorder is a men affected syndrome which causes the deficiency in sexual activity.
Procedure
1. Running the Zymogram
- Prepare 1X Running Buffer, Renaturing Buffer, and Developing Buffer by combining 100 mL of their respective 10X solutions with 900 mL of deionzied water into a new, 1-L autoclaved bottle
- Mix at least 15 uL of each samples 1:1 (vol:vol) with at least 15 uL of Sample Buffer (2X) and allow to sit 5 minutes at room temperature.
- Remove the gelatin zymogram from its package and rinse it with distilled water
- Remove the comb from the top of the gel and the tape from the bottom
- Rinse the wells of the zymogram three times with Running Buffer (1X)
- Assemble the electrophoresis chamber with the zymogram according to manufacturer’s instructions.
- Fill the middle chamber with Running Buffer (1X) until the buffer starts to spill over the top edges into the outer chamber.
- Load 20 uL of samples and protein standards into the zymogram wells using gel loading pipette tips.
- Run the gel for 20 min at 60 V (stack the protein) and then 1.5 h at 125 V. Check for bubbles to ensure that the current is flowing. Keep an eye on the blue dye in each sample to ensure that the proteins are migrating as expected. The blue dye should reach the bottom of the gel at the end of the run.
2. Developing the Zymogram
- Pry open the cassette containing the zymogram with the gel knife (let the opening where the comb was removed face upwards and the other side rest against the lab bench).
- Carefully score the edges of the gel with the gel knife, and cut away the bottom part of the gel to free it from the cassette. Mark the bottom-right side of the zymogram by cutting away at the corner. The cut should correspond to the leftmost side of the gel while loading samples.
- Transfer the zymogram to the top of a sheet-protector. Scan the gel to record the position of the colored molecular weight protein standards.
- Transfer the zymogram to a container and fill it with 100 mL of Renaturing Buffer. Incubate under gentle agitation for 30 min.
- Remove the Renaturing Buffer and add 100 mL of Developing Buffer. Incubate under gentle agitation for 30 min.
- Remove the Developing Buffer and add another 100 mL of Developing Buffer.
- Place the zymogram in the humidified incubator set to 37°C. Incubate it overnight.
- Note: The incubation time can be varied as necessary to increase/decrease the sensitivity of zymography (longer incubation periods allow for more sensitive detection of MMPs).
The next day…
8. Remove the Developing Buffer and replace it with 100 mL of deionized water. Wash under gentle agitation for 5 minutes.
9. Wash a second time under gentle agitation for 5 min with a fresh 100 mL of deionized water.
10. Wash a third time under gentle agitation for 5 min with a fresh 100 mL of deionized water.
11. Remove the deionized water and stain the gel under gentle agitation overnight with 40 mL of SimplyBlue SafeStain.
- Note: Some protocols call for using only 20 mL of staining solution for 1 hour, followed by a couple of washes in water to de-stain the gels. However, I have found that using a lower volume and shorter staining time period results in a lightly-stained zymogram that is harder to interpret.
The next day…
12. Transfer the zymogram to the top of a sheet-protector and scan the gel to record the size and position of the clear bands.
3. Interpretation of the Zymogram
- The bands of clarity indicate regions where MMPs have cleaved the gelatin in the zymogram.
- The position of the clear bands can be used with the molecular weight protein standard to identify the molecular weight of the MMPs that cleaved the gelatin at that location. See this post for our list of possible MMPs that can correspond to each molecular weight.
- The size of the clear bands can be measured in ImageJ in order to semi-quantify the relative amount of MMPs in each sample.
References
- Hu, X., Beeton, C. “Detection of Functional Matrix Metalloproteinases by Zymography.” J. Vis. Exp. (45), e2445, DOI: 10.3791/2445 (2010).
- Invitrogen. “Novex Pre-Cast Gel Electrophoresis Guide” <http://tools.invitrogen.com/content/sfs/manuals/electrophoresisguide_man.pdf>
Citing this Protocol
Farhat, Y. “Gelatin Zymography Protocol.” The Protocol Place. Oct 2012. Accessed on mm/dd/yyyy. <http://protocol-place.com/>
